Home / Resource Center / Application Notes / Milling affects volatile compound recovery in functional mushroom extracts
Milling affects volatile compound recovery in functional mushroom extracts
Dingding Xuan, Sajni Shah, Dr. Eric Janusson, Dr. Markus Roggen- Controlled Chemistry
Introduction
Fritsch milling equipment is an alternative to uncontrolled milling for sample preparation. Here we show
a general method and assay for volatile constituents of functional mushroom extracts can be
dramatically affected by milling conditions
Hand grinders, food processors, coffee grinders, and other milling methods with limited control settings
are standard in laboratories. However, many biomolecules of interest are labile and could be consumed
during these processes. Therefore, instruments that provide little control of milling parameters may
yield inaccurate results for compound discovery and quantification. We investigated whether particle
size, mill time, and blade speed influence analyte recovery and subsequent measurement of compound
concentration in functional mushrooms. We used Lion’s Mane (Hericium erinaceus) as the test
substrate and a Fritsch P11 bladed mill to precisely control the various milling factors. Using the most
abundant components of these mushrooms (leucine, myrcene, alpha-hydroxyisobutyric acid, 2-
acetylbutyrolactone, 2-diethylaminoethyl acetate, and mannitol), we monitored their concentration
resulting from various milling procedures.
Experimental
Lion’s Mane mushroom extracts were prepared through a simple sample preparation and extraction
analytical workflow. Experimental variables were minimized to emphasize differences in milling
conditions. The extracts were surveyed by GCMS for compound discovery and quantification.
Sample Preparation
Vacuum-dried whole Lion’s Mane mushrooms were donated by Nammex (Gibsons, BC). Whole, dried,
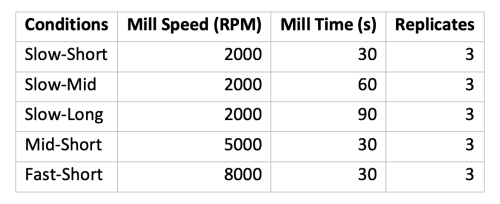
Lion’s Mane mushrooms were weighed, sectioned using a razor, milled using a Fritsch P11 bladed mill,
and then run through a 7mm particle filter. Batches of material were milled at various rates
programmed into the P11 mill (see milling parameters in Table 1). An aliquot of each batch of milled
material was taken for particle size analysis and a separate aliquot was taken for liquid extraction.
Extraction
Milled Lion’s Mane (1.0g) was weighed into 20mL scintillation vials. The extraction was performed with
20.0 mL of a 1:1 mixture of HPLC grade methanol and deionized water containing 0.5% (v/v%) formic
acid (Fisher Optima) at room temperature for 3 hours. The extracts were decanted, and an aliquot was
filtered with 0.20 µm Nylon syringe filters into glass vials for analysis.
GCMS Analysis
The samples were analyzed by GCMS (Agilent Intuvo 9000 GC, 5975 MSD) using an oven ramp program
designed to survey a broad range of analytes (Table 2). The injection volume was 1.0 µL and the GC
column used was an HP-5ms ultra inert 30 m fused silica column. Suitable compounds were extracted
from the GCMS raw data and identified using the Agile 2 peak identification algorithm. Compound
fragmentation patterns, extracted ion areas, and retention times were extracted using MS-DIAL and
assigned through comparison to our proprietary spectral database.
Results
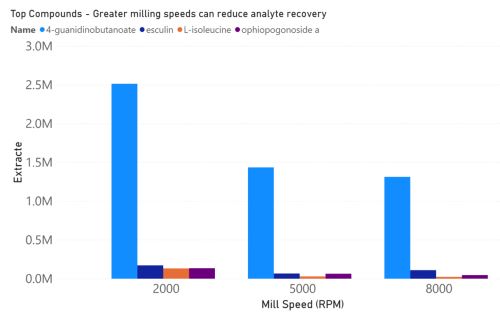
Conclusions
The experiments above demonstrate that precise control of mill conditions is important in mushroom
sample preparation. Lion’s mane mushroom samples were milled with a Fritsch P11 mill set to different
parameters of mill speed and time, and then underwent liquid extraction. The analyte composition of
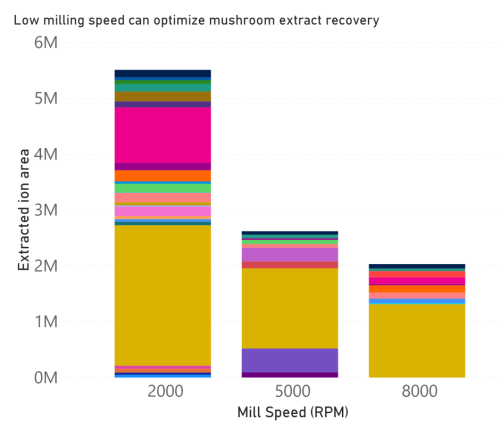
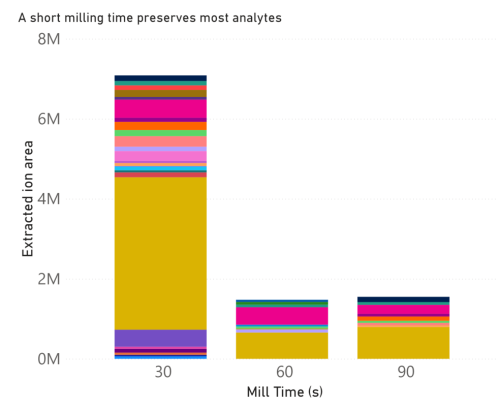
the extracts was then identified as shown in figures 1 and 2. Our results show that mill speed and time
correspond to changes in mushroom extract analyte composition. We could show that mill settings had
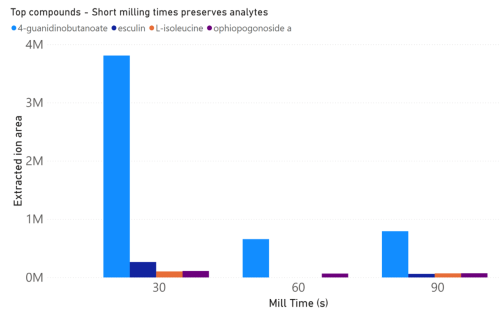
a direct influence on analyte recovery from the mushroom (figures 3 and 4). 4-guanidinobutyrate is
found in edible mushrooms and is a known fungal metabolite in many species.1,2 Hericium erinaceus is
known to have 3.3% of its protein content made up of L-isoleucine.
3 Meanwhile, to the best of our
knowledge, esculin and Ophiopogonoside A have not yet been described in these mushrooms. Among
these compounds, lower mill speeds and shorter mill times corresponded to increased recovery. This
indicates a Fritsch P11 mill would allow researchers to optimize their sample prep to ensure the highest
yields.
References
- PubChem Compound Summary for CID 500, 4-Guanidinobutyric acid.
National Center for Biotechnology Information
https://pubchem.ncbi.nlm.nih.gov/compound/4-Guanidinobutyric-acid (2022). - Li, J., Wu, H., Wang, L., Huang, Y. & Wang, L. Key taste components in two wild edible Boletus mushrooms using widely targeted metabolomics.
Biochemical Systematics and Ecology 96, 104268 (2021). - Aparicio-Razo, M. & González-Pérez, M. ANALYSIS OF BIOMOLECULES OF THE FUNGUS HERICIUM ERINACEUS THROUGH THE THEORY OF ELECTRON
TRANSFER OF QUANTUM CHEMISTRY AND ITS RELATIONSHIP WITH THE PRIMARY AMINO ACIDS. 9, 139–147 (2020).
Supporting
Table 2
| Name | Abundance in all experiments |
|---|---|
| 4-guanidinobutanoate | 5261052.235 |
| L-leucine | 1110933.091 |
| 3-amino-1,2-propanediol | 425572.75 |
| esculin | 345937.8184 |
| L-beta-homoleucine | 326320.0547 |
| tetrahydrofuran | 272256.9375 |
| dodecamethylcyclohexasiloxane | 270464.666 |
| cadaverine | 244824.6094 |
| ophiopogonoside A | 243731.9395 |
| hexazinone | 240513.2041 |
| citropen | 223926.4727 |
| 4-oxo-3-phenyl-6-propyl-4h-chromen-7-yl acetate | 195539.752 |
| L-isoleucine | 183783.2715 |
| dehydroandrographolide | 181819.543 |
| N,N-dibutylnitrous amide | 176344.2031 |
| myrcene | 134515.9443 |
| 4-hydroxybenzaldehyde | 122221.9766 |
| alpha-hydroxyisobutyric acid | 120649.4336 |
| N,N’-dicyclohexylthiourea | 112791.6953 |
| 2-hydroxybutyric acid | 94918.07813 |
| 2-((7-acetamido-1,2,3-trimethoxy-9-oxo-5,6,7,9- tetrahydrobenzo[a]heptalen-10-yl)amino)-n-(4-(5-(methoxymethyl)- 1h-1,2,4-triazol-3-yl)phenyl)acetamide | 90886.3457 |
| cyclododecanol | 86997.33765 |
| 6-methoxy-4-methyl-2h-chromen-2-one | 82209.84376 |
| phenaceturic acid | 66982.5 |
| 1-myristoyl-2-hydroxy-sn-glycero-3-phosphate (sodium salt) sodium salt | 66748.45361 |
| 1-[(2e,4e)-6,7-dihydroxy-2,4-octadienoyl]prolyl-n-methylvalyl-n2- methylalaninamide | 62692.64649 |
| butanal | 54538.58203 |
| pyruvaldehyde | 53863.91406 |
| 2-methylbutyrylglycine | 53269.54297 |
| cinchonine | 52899.89649 |
| sinapic acid | 48724.09424 |
| diffractaic acid | 44918.83301 |
| l-2,3-diaminopropionic acid | 38335.02344 |
| [(2r,3r,4s,5r,6s)-3,4,5-tris(acetyloxy)-6-[(7-tert-butyl-5,8-dihydroxy1,4-dioxo-3-{[(2s,3r,4s,5r,6r)-3,4,5-tris(acetyloxy)-6- [(acetyloxy)methyl]oxan-2-yl]sulfanyl}-1,4-dihydronaphthalen-2- yl)sulfanyl]oxan-2-yl]methyl acetate | 36675.40137 |
| pseudolaric acid a-o-beta-d-glucopyranoside | 31419.36426 |
| dl-coniine | 28856.19677 |
| ganoderol b | 28060.43164 |
| cyanate | 25044.28906 |
| allo-threonine | 23335.97266 |
| 4-acetamidobutanoate | 22061.03906 |
| nerolidol | 22051.06641 |
| pseudopyronine b | 21448.30469 |
| dioctyl phthalate | 21001.31372 |
| daphnetin | 20752.16382 |
| mannitol | 19570.46094 |
| protocetraric acid | 18046.0166 |
| octylamine | 16933.83765 |
| n-acetyl-b-alanine | 16850.11523 |
| n-octyl-2-pyrrolidone | 16671.71484 |
| L-norleucine | 14774.7041 |
| flucarbazone | 14044.42285 |
| isoamylamine | 12573.57324 |
| bis(2-ethylhexyl) phthalate | 12386.82568 |
| 2-amino-3-methylvaleric acid | 11756.65332 |
| formiminoaspartate | 11709.78027 |
| 1-dodecyl-2-pyrrolidinone | 11695.82129 |
| sakuranetin | 11362.86719 |
| 3-methyl-2-oxobutanoate | 11235.17969 |
| 1-dodecanamine | 11142.62036 |
| isocucurbitacin b | 10466.6709 |
| guanidine | 10229.51465 |
| lutein | 9612.479492 |
| benzoic acid | 9459.237305 |
| lacosamide | 8988.265625 |
| dimethyl malate | 8386.848145 |
| 1,2-dilauroyl-sn-glycero-3-phosphate monosodium salt | 5801.692871 |
| norfluoxetin | 5688.64502 |
| metformin | 5674.167969 |
| loureirin a | 5657.355713 |
| trimethylamine | 5535.165039 |
| mexiletine acetate | 5474.612305 |
| 1-myristoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine | 5455.387695 |
| norfluoxetine | 4984.3125 |
| dl-norgestrel | 3903.630127 |
| aminoadipic acid | 3554.445068 |
| ethanolamine | 2852.979736 |
| diisopropyl phthalate | 2370.876465 |
| 4-oxobutanoate | 1833.481812 |
| 1,5-diaminopentane | 268.306152 |
Post Views: 211
